Cyber security risk sparks implanted device recalls
Modern advances in digital health monitoring – including implantable sensors to detect irregular heartbeats, for example – are changing the way we manage our health.
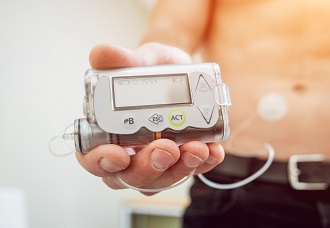
These implanted devices are programmed wirelessly, and are able to transmit information to your doctor or to other devices, such as external blood sugar monitors which control how much insulin an implanted pump releases.
However, it’s unclear if the devices are safe from hackers who may be able to illegally access your information, steal your identity, or even make the implanted device malfunction.
The FDA is warning that certain insulin pumps – the Medtronic MiniMed 508 series and MiniMed Paradigm series pumps – are at risk of being hacked. Medtronic recalled the mentioned pumps earlier this year, estimating that at least 4,000 people are at risk. The FDA stated that Medtronic would provide alternative pumps.
If you have one of the above devices, talk to your doctor to get the process started or if you are concerned that your pump’s settings have been changed.
In March, the U.S. Department of Homeland Security issued similar warnings about 750,000 Medtronic implantable cardioverter-defibrillators. Medtronic did not recall them but is instead working on a software update in order to improve security.
Tags: device, Health


